Determining Strong and Total Alkalinity in Water By Hanna Titration System
 Alkalinity is a measure of a water sample’s ability to resist a pH change when reacted with an acid; and is an important measure of water quality. If a body of water has high alkalinity, it is highly resistant to changes in pH due to acid additions such as acid rain or industrial waste discharge. Conversely, low alkalinity means a body of water is susceptible to rapid pH changes, even from small acid additions. Water in industries is consumed for boiler make-up, processing, product treatment and cleaning, cooling, etc. The quality level required for chemical, food, textile and pulp/paper industry is high, therefore measurement of water quality is highly important.
Alkalinity is a measure of a water sample’s ability to resist a pH change when reacted with an acid; and is an important measure of water quality. If a body of water has high alkalinity, it is highly resistant to changes in pH due to acid additions such as acid rain or industrial waste discharge. Conversely, low alkalinity means a body of water is susceptible to rapid pH changes, even from small acid additions. Water in industries is consumed for boiler make-up, processing, product treatment and cleaning, cooling, etc. The quality level required for chemical, food, textile and pulp/paper industry is high, therefore measurement of water quality is highly important.
Titration is the most common method used to measure alkalinity. While simple in theory, multiple variations of alkalinity testing can be performed. Each test variation yields different information about the sample. For example, above pH 8.3, alkalinity is present predominantly as carbonate (CO₃²¯) and hydroxide (OH¯) salts. Between pH 4.5 and 8.3, alkalinity is present mostly as bicarbonate (HCO₃¯) salts. At pH 4.5, the buffering capacity of these salts is exhausted, and these forms are almost completely converted to carbonic acid (H₂CO₃). An analyst can determine these various forms of alkalinity by adjusting the titration endpoint, as different alkalinity constituents are present in different proportions depending on pH. A total alkalinity titration is commonly performed by adding hydrochloric acid titrant to the sample until reaching a pH 4.5 endpoint. The total alkalinity result incorporates contributions from all alkalinity forms (hydroxide, carbonate, and bicarbonate ion). A strong alkalinity test is performed by titrating the sample until a pH 8.3 endpoint. At this pH, alkalinity is converted from carbonate form to bicarbonate form. The strong alkalinity result incorporates alkalinity contributed by some of the carbonate present and hydroxide, but not bicarbonate. Strong alkalinity can only be present if the starting pH is above pH 8.3; otherwise, only weak alkalinity (alkalinity between pH 8.3 and pH 4.5) is present.
Application
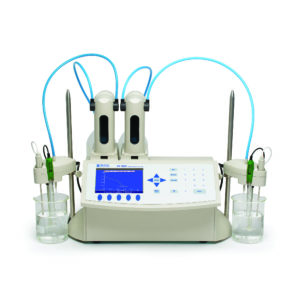
A Consumer Product Manufacturer (Refrigerator/Washing Machine) contacted Hanna Equipment’s that they are interested in testing strong and total alkalinity quickly and easily. The customer wanted an automatic titrator to reduce the time and monotony associated with manual titrations; this would allow technicians to use their time more effectively on other tasks and increase throughput. The laboratory ran 30 alkalinity samples a day for both strong and total alkalinity. Hanna Equipment’s suggested the HI902C Automatic Potentiometric Titrator.
The customer often ran strong and total alkalinity on the same sample, so Hanna Instruments suggested the use of a linked method with The use of linked methods allowed the customer to run multiple analyses on the same sample without interruption.
The HI902C is an automatic titrator that complements our wide range of products dedicated to efficient and accurate laboratory analysis. The HI902C potentiometric titrator can perform acid/base, redox (ORP), complexometric, precipitation, non-aqueous, argentometric, and ion selective titrations, as well as back titrations and titre determinations. This powerful titrator automatically dispenses the titrant, detects the endpoint, and performs all necessary calculations and graphing. In addition to titration, the HI902C also operates as a fully functional pH, mV/ORP, and ion selective electrode (ISE) meter.
To know more,
Visit : https://hannainst.in/hi902c1-02-automatic-titration-system.html
mail at marketing@hannainst.in



